 苏博科从2012年起担任阿斯利康首席执行官。图片来源:ZACH GIBSON—BLOOMBERG VIA GETTY IMAGES
苏博科从2012年起担任阿斯利康首席执行官。图片来源:ZACH GIBSON—BLOOMBERG VIA GETTY IMAGES6月底一个星期三的早上,南非约翰内斯堡郊外黑人聚居的索韦托,小姆隆戈走进克里斯·哈尼·巴拉瓜纳医院。他坐在诊室里解开紫色夹克衫拉链,因为此时南半球正是冬天。为了避免感染新冠病毒,他一直戴着红色布口罩。
姆隆戈从外套袖子里伸出左臂,拉起衬衫袖口。护士把针扎进胳膊注射透明液体时,他有点紧张。“我有点害怕,但我想了解这种疫苗,然后告诉亲朋好友,”他对身旁围观的记者说。
等待答案不仅仅是姆隆戈,全世界都在等。这位年轻的志愿者是南非首批约2000名患者之一,测试由英国牛津大学科学家研发的新冠疫苗,英国、美国和巴西都有数千人参与。
疫苗是终结新冠病毒全球大流行的关键。如果成功,经济就能重归增长,也可以拯救无数生命。没有疫苗,数以百万计的人可能会失去生命,企业和政府也会一直笼罩在病毒的阴影中。
根据世界卫生组织的数据,截至7月底,至少有168种候选疫苗处于研发阶段。但牛津疫苗在人体试验方面可以说进展最快。初步的人体试验显示颇有希望。为了推动进展,美国政府提前订购了3亿剂,耗资10亿美元。英国订购了1亿剂,欧盟订购了4亿剂,还达成协议向发展中国家提供超过13亿剂。
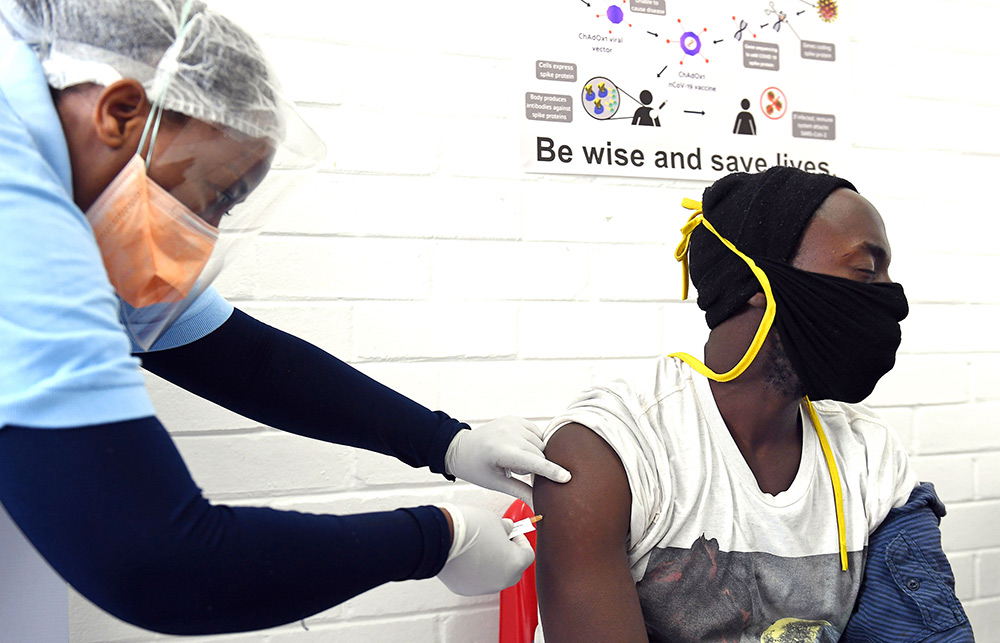 救命一针:南非索韦托一家医院里的志愿者正在接受注射,这是牛津大学詹纳研究所开发的新冠疫苗临床试验一部分。如果试验成功,阿斯利康将批量生产数十亿剂。图片来源:FELIX DLANGAMANDLA—BEELD/GALLO IMAGES VIA GETTY IMAGES
救命一针:南非索韦托一家医院里的志愿者正在接受注射,这是牛津大学詹纳研究所开发的新冠疫苗临床试验一部分。如果试验成功,阿斯利康将批量生产数十亿剂。图片来源:FELIX DLANGAMANDLA—BEELD/GALLO IMAGES VIA GETTY IMAGES尽管如此,疫苗研发始终是一场赌博。麻省理工学院在2018年的研究发现,传染病疫苗研发失败的几率达66%。英国制药巨头阿斯利康的首席执行官苏博科(Pascal Soriot)大手笔下注牛津研发的疫苗。4月底,苏博科果断出手,阿斯利康击败疫苗研发方面成绩更好的竞争对手,携手牛津成为新冠疫苗商业合作伙伴,争取在全球研发竞赛中领先。
与牛津合作引起了外界对阿斯利康的极大关注。但这只是过去八年里阿斯利康一系列大胆合作、战略转型和谨慎冒险举动的最新一步,正是因为各种举措阿斯利康才能从大药企里最不成功的药物研发公司发展为最可靠的巨头。苏博科是推动转型的工程师。这位61岁的药剂师出生于法国,虽然大部分职业生涯都在国外,英语里还是总带着法语味。他头发乌黑,与喜欢的清爽白色衬衫形成鲜明对比,他说话非常直率,在高管圈中相当少见。苏博科告诉《财富》杂志,他信奉“轻松又紧张”的氛围。“我们必须认真对待工作,但不应该把自己看得过重。”他说。
当然,很少有制药公司的首席执行官面临着新冠病毒一样严重的生存挑战。与牛津大学的合作存在风险,因此阿斯利康抽调数百名员工在疫苗被证实有效之前就扩大临床试验和生产。至于疫苗是否真的有效,要到今年秋天才能知道。但如果临床试验得到积极的结果,美国和英国监管机构有望紧急批准,苏博科承诺尽快在9月下旬开始供应,从而开始大规模接种。
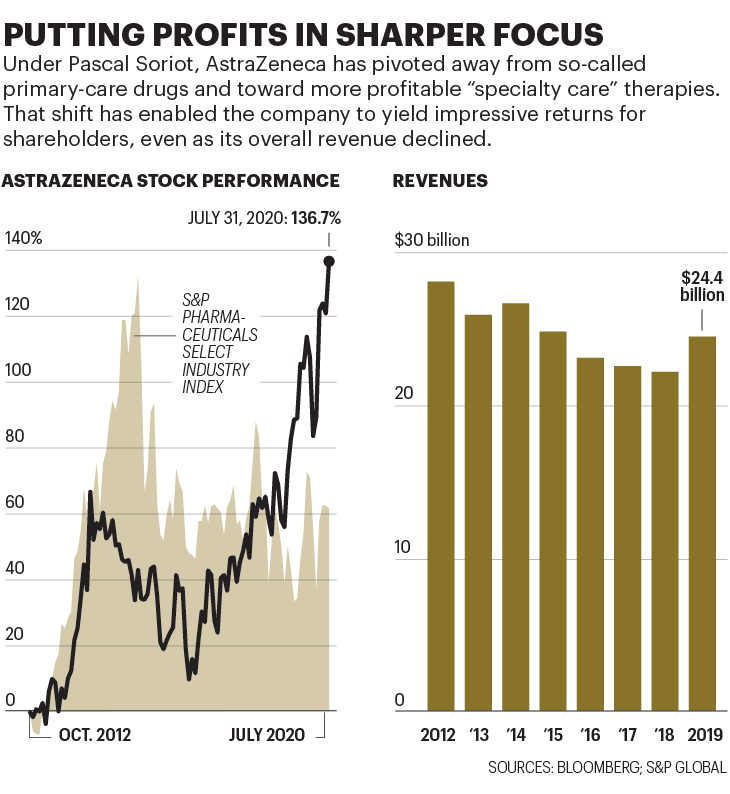
平均来说,研发一款新疫苗需要10年以上的时间。这次却只有6个月。即使成功,去年收入244亿美元的阿斯利康一开始也不会赚钱。因为阿斯利康已经同意以成本价提供最开始的20亿剂疫苗,还有可能提供更多。只有证明新冠是流感一样地区性季节性的威胁,人们需要定期接种疫苗,才能提高阿斯利康的利润。不过,股市上阿斯利康已经暴涨。宣布与牛津合作的当天,公司股价飙升至有史以来最高。之后股价最高达到97英镑(124美元),比疫情前上涨了38%,目前阿斯利康是伦敦富时100指数里市值最高的公司。
至于阿斯利康如何获得牛津疫苗的内幕消息,跟所有商业故事一样也是靠关系。但同样关系到新冠病毒令人担心的地缘政治。这是非凡的企业转型故事,为关注科学前沿希望获利的企业以及想推进激进文化变革的高管提供了经验教训。
去年12月,中国武汉出现神秘呼吸道疾病的消息刚浮出水面,立刻引起了阿斯利康注意。与其他西方制药公司相比,阿斯利康在中国的业务规模更大。公司6.15万名员工中,近四分之一常驻中国,去年公司在华收入约48亿美元。从去年12月底开始,苏博科几乎每天都与公司驻中国的高管视频通话,跟踪疫情发展。
“我们很快就开始考虑,怎样才能提供帮助?”苏博科回忆道。阿斯利康购买了900万台可以过滤病毒的FFP3呼吸机,捐赠给世界各地的医护工作者。公司开始调查现有药物,包括抗炎症的白血病治疗手段和保护心脏和肾脏的糖尿病药物能否用于抑制新冠病毒。阿斯利康也与范德比尔特大学合作研究可能用于治疗的合成抗体。当英国检测新冠病毒能力明显缺乏时,阿斯利康与英国竞争对手葛兰素史克和剑桥大学合作成立了新的检测实验室。
阿斯利康的应对手段中明显缺少一块:疫苗。不过疫苗领域并不是阿斯利康的强项。目前公司只销售一种疫苗,预防季节性流感的鼻喷雾。但在离剑桥大学总部不远的对手大学里,事件即将出现变化。
詹纳研究所位于牛津大学一组研究实验室,坐落在低矮的现代化办公楼里,装有烟灰色玻璃窗和绿色保护层。研究所以18世纪第一位为人们接种天花疫苗的爱德华·詹纳医生名字命名,过去20年里,此地已经成为全世界最重要的疫苗研发中心之一。
牛津大学之所以能在冠状病毒疫苗研发中领先,主要因为本次的冠状病毒与引起非典(SARS)和中东呼吸综合症(MERS)的冠状病毒有相似之处。詹纳研究所的研究员莎拉·吉尔伯特一直在研究中东呼吸综合症疫苗,她很快意识到有可能用同样的方法治疗新冠病毒。
大多数疫苗都是用相应病毒降低毒性后制成。吉尔伯特的疫苗则有所不同,利用无害的黑猩猩病毒并采取基因改造,产生新冠病毒表面的刺突蛋白,从而使身体产生潜在的有效免疫反应。如此生产的疫苗至少有一项主要优势,即不必低温保存,这在缺乏可靠冷链运输和储存手段的发展中国家尤为重要。
牛津大学的科学家们通过先前的研究了解到,改良的黑猩猩病毒是安全的,也知道可以大批量生产。但协助监督牛津医学部与外部合作伙伴合作的教授约翰·贝尔说,牛津大学更深知需要大型制药公司帮助。“说明一点,为全世界制造数十亿剂量的疫苗,不是大学能做到或应该做的。”贝尔表示。68岁的贝尔是加拿大人,也是运动健将,习惯把眼镜顶在银灰色头发上。

牛津大学开始寻找了解疫苗的合作伙伴,但政治因素导致谈判变得困难。美国一些公司坚持获得全球独家生产权,牛津对此犹豫不决。特朗普政府准备提供大笔资金,确保美国公众能首先买到疫苗。美国、法国和德国等国政府都发誓阻止本国生产的疫苗出口。贝尔说,每年向牛津大学提供数百万英镑资金的英国政府越来越担心,最终可能得不到足够的疫苗接种,于是4月通知牛津大学寻找英国合作伙伴。
英国只有两家公司生产能力达标,就是葛兰素史克和阿斯利康。选择葛兰素史克似乎无需多考虑。葛兰素史克已经是全世界最大的疫苗生产商,拥有超过30种疫苗,包括麻疹、脑膜炎和肺炎疫苗。不过,葛兰素史克已经在自行研发新冠疫苗,包括与法国制药巨头赛诺菲和中国生物技术公司三叶草生物制药的合作。(与三叶草合作项目正处于早期人体试验阶段。)
阿斯利康之前几乎没有生产过疫苗。但其生产工艺与吉尔伯特改良黑猩猩病毒的过程颇为相似。(阿斯利康用来生产合成抗体。)更重要的是,贝尔与苏博科有私交。贝尔曾经在瑞士制药商罗氏的董事会任职,去年才离开。而苏博科曾经在罗氏表现优异,他在去阿斯利康担任最高职位之前曾经于罗氏担任两年首席运营官。“我知道苏博科追求最尖端的科学,也有勇气冒险。”贝尔说。“而且老实说,这种疫苗风险很高。”
牛津还考虑到了另一项重要因素:阿斯利康在苏博科的领导下研发领域的进展令人瞩目。“这是过去20年里制药公司东山再起的故事之一。”贝尔说。瑞银的股票分析师迈克尔·莱克敦斯在谈到阿斯利康的复苏时说:“很了不起。我以前从未见过,以后应该也难得一见。”
2012年,苏博科执掌阿斯利康时,发展前景相当暗淡。公司最畅销的高胆固醇药瑞舒伐他汀、治疗胃酸倒流的耐信和抗精神病药物喹硫平都即将跌落可怕的“专利悬崖”。一旦失去知识产权保护,仿制药制造商就能销售廉价的版本。阿斯利康年销售额的一半可能在五年内消失,金额达170亿美元。
更糟糕的是,公司在研发方面投入不足,无法推出新药代替原有畅销药。“当时,公司就像电子表格一样运转。”苏博科说。“整天想着节省成本增加利润,把利润用于回购,机械地提高股价。但公司没有前途。”很多投资者都同意。“他必须重新考虑战略。”当苏博科上任时,资产管理公司AGF的基金经理斯蒂芬妮·马厄这样表示。
苏博科刚一接手,就宣布停止回购,将资金投入研发。2011年,苏博科获任命前一年,阿斯利康的研发支出仅占销售额16%;到2019年,研发支出已占销售额26%,是同行当中最高的。不过,要想恢复药品生产线,不能遇到问题就投入资金解决,关键在于重新调整全公司的重点。
十年前,阿斯利康研发运作效率极低,只有4%的研发药物最终能面世。“每年在研发上花费50亿美元,却没有做出新药。”阿斯利康的生物制药研发负责人梅内·潘加洛斯说。2012年,咨询公司InnoThink生物制药创新研究中心的一项发现证据确凿,研究发现阿斯利康旗下获得美国食品和药监局批准的新药平均研发支出高达118亿美元,在业内是最差记录。
 公司战略转变之前,“我们每年在研发上花费50亿美元,却没有做出新药。”阿斯利康的生物制药研发负责人梅内·潘加洛斯说。图片来源:COURTESY OF ASTRAZENECA
公司战略转变之前,“我们每年在研发上花费50亿美元,却没有做出新药。”阿斯利康的生物制药研发负责人梅内·潘加洛斯说。图片来源:COURTESY OF ASTRAZENECA苏博科的前任大卫·布伦南聘请潘加洛斯重振研究部门。苏博科接手后,加快了新药研发节奏。先前一次领导层会议上,一位销售主管说有位研究员的介绍“有点偏技术,我都听不懂。”苏博科公开提出指责。“如果你想成为高层领导,就要对我们的研究还有治疗的病人真正感兴趣然后真心投入。”潘加洛斯回忆道。“一片寂静,有几个人咽了咽口水。但如果是在场的研发人员,感觉相当好。”
改革刚刚起步就差点胎死腹中。2014年春,美国规模更大的制药商辉瑞发起了恶意收购。首席执行官回忆说,刚开始投资银行家最初告诉苏博科,公司保持独立的可能性不到10%。苏博科全力抵抗,并向投资者承诺,到2023年将维持股息增长,收入提高到400亿美元,增幅达75%。苏博科说,回想起来当时的收购激发了他的战略,也促使全公司清楚地认识到,要生存就要靠更有效也更能赚钱的研发。
阿斯利康一直主要研发初级保健药物,治疗过敏和高血压等慢性疾病。苏博科将重点转移到专科护理药物上,此类药要求保险公司提供高额补偿,利润率较高,尽管市场规模较小。
肿瘤学方面的调整最引人注目。在苏博科前任的领导下,阿斯利康逐步放弃癌症治疗领域。但之前公司外部重要的肿瘤学家告诉苏博科,阿斯利康公司即将停产治疗晚期卵巢癌的药利普卓可能畅销,因为临床试验结果显示有希望。于是苏博科调整方向,恢复了该药生产。
这是一个重大决定。利普卓是名为PARP抑制剂的药物先驱。此类药针对细胞修复至关重要的酶,通过阻断酶可以阻止癌细胞恢复活力。研究很快发现,利普卓也能够治疗部分胰腺癌、乳腺癌和前列腺癌。现在利普卓是最畅销的PARP抑制剂,2019年创造了12亿美元收入,销售额同比增长85%。其功能多样性也吸引了企业合作伙伴。2017年,默沙东同意向阿斯利康支付高达85亿美元,以共同研发利普卓。
利普卓是阿斯利康肿瘤战略的代表案例,在该战略的指导下,公司研发出相当专业的药物,价格也相当高。泰瑞沙针对特殊且高度危险的肺癌突变,已经成为阿斯利康最耀眼的明星,仅去年就带来32亿美元收入。英飞凡是治疗肺癌、泌尿癌和膀胱癌的免疫疗法,2019年收入15亿美元。(阿斯利康还就肿瘤药物寻求合作,与日本第一三共株式会社签署了两项数十亿美元的协议。今年7月,阿斯利康同意支付高达60亿美元,与第一三共合作开发可限制化疗副作用的药物。)肿瘤药目前占阿斯利康年销售额37%,超过其他领域,远高于五年前的占比12%。
除了肿瘤,苏博科还围绕心血管、肾脏、代谢疾病、呼吸系统疾病和免疫学药物重组了阿斯利康。各种类别中,苏博科和潘加洛斯建立了严格程序评估候选药物。该程序又称为“五R框架”,要求开发药物之前必须确定五个方向“准确”:准确的目标、准确的患者群体、准确的身体组织、准确的安全制度,以及最关键的准确商业机会。清单听起来像制药行业基础知识,但之前阿斯利康很缺乏相关纪律。
严格的部署大大减少了阿斯利康研发药物的数量。但由于将有希望的分子从临床前研究转移到完成晚期临床试验,成功率显著提高。之前成功率仅为4%,现在是20%,是行业平均水平的三倍。
阿斯利康还利用新数字技术重新设计临床试验方法。研发部门的首席数字健康官克里斯蒂娜·杜兰表示,Merlin的软件能协助更快选择试验地点。而且几分钟内Merlin就可以估算出试验成本(过去需要几天)。有个研究生态系统里,受试者往往严重偏向男性和白人,Merlin则帮助选择更能反映真实世界人口的试验人群。还有个叫“控制塔”的软件系统,管理人员可在数据面板上一次性获得阿斯利康所有相关试验的快照,从而预测招募患者时可能出现的问题。“之前的系统我们已经用了20年,过去两年里全盘改变,很疯狂也很勇敢,但最后成功了。”杜兰说。
为了强调以科学为导向,2016年苏博科将位于伦敦的公司总部和位于麦克斯菲尔德的研究总部迁至剑桥。苏博科正在监督建设造价12亿美元的战略研发中心地标建筑,外形为圆形玻璃甜甜圈,可以展示阿斯利康科学家的工作。建筑距离被称为“诺贝尔工厂”(已经荣获12次)的政府分子生物学实验室,还有顶尖的癌症和干细胞研究实验室只有几步之遥。迁址以来,阿斯利康离开与剑桥大学的合作从五年前的不到10个增加到130多个。
如今,阿斯利康公司的规模比改造前要小,主要因为原先畅销药失去专利保护后的销售损失。去年,阿斯利康收入比2011年下降了27%。但投资者似乎认为,药品质量弥补了销售数量上的不足。自苏博科接手以来,以美元计算,阿斯利康的股价已经上涨137%,轻松跑赢同期标普医药精选行业指数61%的涨幅。
4月最后一周,阿斯利康生产牛津疫苗的交易在一连串电话会议中促成。牛津大学提出了两个条件:疫情结束之前阿斯利康放弃疫苗的利润,承诺尽可能广泛地提供疫苗。两项阿斯利康都同意。
对阿斯利康来说,疫苗是形象项目,可以证明公司有能力与科学前沿的学术团体和生物技术公司合作。这比吹嘘专利权更有价值,相关合作关系对阿斯利康的药品生产线至关重要。与此同时,疫苗项目速度也可以测试阿斯利康为推进更高效临床试验建立的系统。
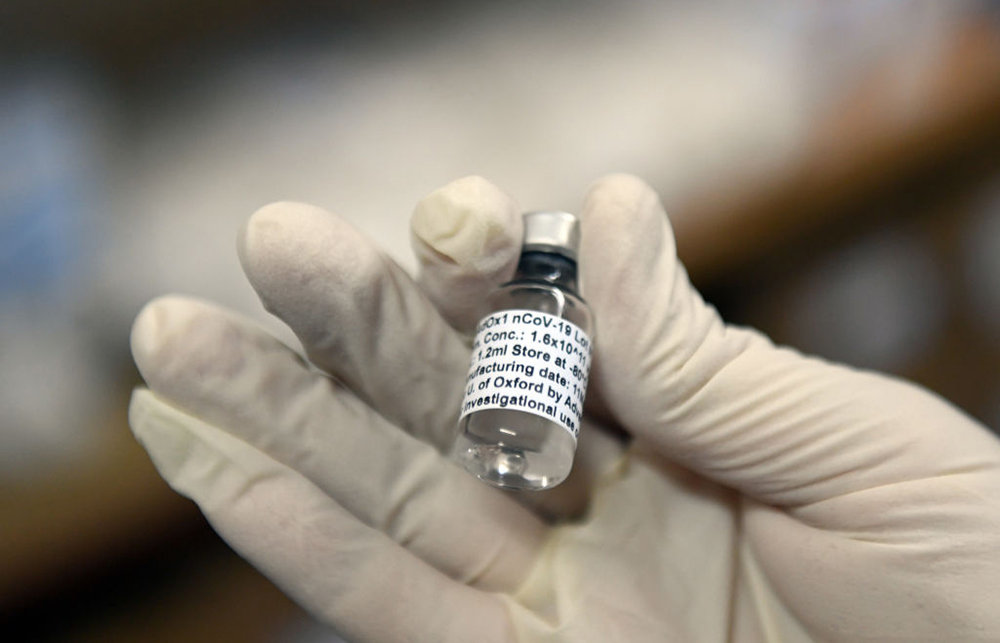 南非索韦托临床试验中,一小瓶牛津大学/阿斯利康合作推出的试验性新冠疫苗。图片来源:FELIX DLANGAMANDLA—BEELD/GALLO IMAGES/GETTY IMAGES
南非索韦托临床试验中,一小瓶牛津大学/阿斯利康合作推出的试验性新冠疫苗。图片来源:FELIX DLANGAMANDLA—BEELD/GALLO IMAGES/GETTY IMAGES为将通常需数年时间的测试浓缩在几个月内完成,牛津大学采取了前所未有的措施:以往第一阶段试验只招募少量病人,目的是通过小规模试验证明药物安全,本次牛津大学招募了1100人,并加快了接种速度。第二阶段的试验是在更多志愿者身上进行安全性和有效性试验,第三阶段试验证明药物效果,三个阶段通常按顺序进行。本次则是同时进行。牛津在英国招募了10000人,还有巴西的5000人和南非的2000人,阿斯利康在美国也招募了30000名志愿者。
苏博科说,预计结果将在9月至11月之间择时公布。但疫情紧迫性意味着试验结束之前就要开始大规模生产疫苗。在各国政府和国际机构资助下,阿斯利康不计利润授权全球企业生产,还与私营生物技术公司印度血清研究所达成里程碑式协议,为中低收入国家提供10亿剂。
6月初,阿斯利康飞涨的股价一度陷入恐慌。彭博新闻社援引匿名消息人士报道称,阿斯利康已经与美国吉利德制药公司商谈超大规模的合并,如果消息属实将成为制药行业历史上规模最大的并购。疫情中吉利德因旗下瑞德西韦家喻户晓,瑞德西韦是少数几款被证明可以减少新冠重症患者住院时间的药物之一。
吉利德拒绝就报道发表评论,苏博科也拒绝置评。不过,他在接受《财富》杂志采访时表示,当前环境下发起并购谈判意义不大。他说:“我只想请大家看清事实。”他还表示,疫情中推进临床试验让人费尽心思。疫苗研制也是如此。“这项任务让大家充满活力,但对很多人来说是艰巨的任务。”此外,考虑到旅行限制和隔离措施,谈判团队都无法面对面。“想象一下史上最大的制药行业并购案通过Zoom视频会议完成?开玩笑吧。”他说。
总体而言,业内人士和股市分析师都认为合并机会很小。不过有人指出,也有一种可能的情况下合并具有合情性,这种可能性会让投资者毛骨悚然,如果阿斯利康因为现金短缺而被迫交易,该怎么办?
吉利德坐拥240亿美元的现金储备。最近阿斯利康取得了成功,股票估值也很高,现金流却很差。去年,公司未能通过经营产生足够净现金支付35亿美元股息,而苏博科抗击辉瑞时承诺现金流可以继续保持增长。后来,公司不得不增发股票筹集35亿美元,这也是20年来阿斯利康为支付股息和履行其他义务首次增发。
阿斯利康现金短缺问题一定程度上源于推动创新。该公司重组费用终止遗留项目,向新药开发合作伙伴一次性预付款,举债为研发提供资金,种种策略都会影响现金流。伦敦Intron Health的分析师纳瑞许·秋汉表示,公司采用了“大量创新会计手段”,即合法但激进的策略,例如,合伙企业存续期间分期偿还一次性付款,防止相关交易降低阿斯利康让投资者注意的非公认会计准则下“每股核心收益”数字。
如此高空走钢丝,让一些投资者保持谨慎。瑞银的洛伊希腾对阿斯利康业绩扭转表示钦佩,但与其他两位分析师(在24位阿斯利康分析师中)对股票给予卖出评级。洛伊希腾称该公司为“概念股”。他说,投资者说服自己,阿斯利康搭建了“平台”生产一系列畅销药,但其表现并不能证明超常估值的合理性,目前公司股价高达每股收益的106倍。
苏博科指责像洛伊希腾之类批评者“眼光短浅到只能看见自己的鞋,看不到地平线。”他说:“人们必须决定的问题是,‘我相信这家公司的未来吗?’”他还为阿斯利康的股息辩护称,耐心的股东自会得到回报。他表示,现金流最终会有所改善。“很快,说‘你应该削减股息’的人转头就会改换批评论调,质问说,‘你为什么不增加股息?’ ”
目前,关于阿斯利康长期财务状况的争论都可以归于一个问题,牛津疫苗能否起效?
7月20日,医学专业杂志《柳叶刀》发表了牛津大学第一阶段试验的结果,答案出现一丝曙光。头条新闻的内容偏正面。(“牛津冠状病毒疫苗首次人体试验显示有望成功。”路透社宣称。),然而消息传出后,阿斯利康的股价下跌5.9%,创下自3月以来最大单日跌幅,随后继续走低。
第一阶段的试验是为了证明疫苗不会威胁使用者的健康,同时也可提供疫苗效果的线索。阿斯利康牛津疫苗似乎在两方面都取得了成功。该药没有严重的副作用。详细分析25名接受过单剂量疫苗接种的志愿者血液后发现,91%的志愿者产生了能中和病毒的抗体。第二次接受增强剂量的10名志愿者中,100%产生抗体,接种者体内抗体水平与感染过新冠病毒又恢复的患者体内抗体水平相当。
那么,市场为何感到不安?部分原因是,第二次使用了加强剂量。结果与第一组结果之间的差异可能说明牛津的疫苗需要两剂。正如潘加洛斯当时指出,大多数竞争中的疫苗研发也在考虑两剂方案。然而,如果需要多次注射,全球疫苗接种计划就会变复杂也更昂贵。如果有种疫苗只需注射一次就能产生免疫效果,可能就会成为首选,从而抵消阿斯利康的先发优势。
牛津大学的疫苗也可能输给两款竞争对手的疫苗。分析人士阅读《柳叶刀》报告后指出,一些竞争疫苗的初步试验显示抗体生成水平高于牛津大学的疫苗,尤其是辉瑞公司与生物技术公司BioNTech合作开发的疫苗。另一个悬而未决的问题是:疫苗是否实现“杀菌免疫”,可以防止接种者将病毒传播给未接种的人。
基于种种原因,牛津大学的贝尔把疫苗成功率定在不超过50/50。“根本不是板上钉钉。”他说。
尽管如此,第一阶段的研究提供信任阿斯利康和牛津团队能成功的理由。抗体数相当高,令人鼓舞。而且到最后新冠病毒抗体可能不是疫苗有效性的唯一关键(事实上越来越多研究表明,抗体可能迅速消退)。阿斯利康和牛津团队积极指出,其疫苗也引发T细胞的强烈反应,T细胞会寻找并消灭病原体。潘加洛斯告诉记者,由于细胞战士表现优异,他“越发相信”牛津疫苗能提供至少一年免疫力。
至于与其他疫苗的强度对比,牛津大学和阿斯利康的研究人员指出,到目前为止,试验采用的血样分析方法各不相同,简单比较不太可能。此外苏博科观察到,市场空间可以容纳不只一个赢家,毕竟要实现全世界安全接种肯定需要多款疫苗。
事实上,牛津大学并不是阿斯利康在新冠疫苗方面的唯一赌注。苏博科出任首席执行官后不久,阿斯利康就向马萨诸塞州剑桥一家生物技术初创公司Moderna投资了2.4亿美元。后来阿斯利康追加了1.4亿美元投资,持股比例增至9%。Moderna正在开发基于信使核糖核酸(mRNA)的疫苗,信使核糖核酸负责将指令从细胞核传达到制造蛋白质的部分。修改信使核糖核酸后,就可以让细胞产生想要的蛋白质,包括(有希望)引起免疫反应的蛋白质。Moderna的疫苗已经进入大规模人体试验阶段,阿斯利康的持股价值也飙升至30多亿美元。
如果运气好的话,两种疫苗都可能成功。现在所有目光都集中在后期试验上。测试需要在新冠病毒感染猖獗的地方进行,科学家就可以比较接种过疫苗的群体和对照组。这就是为何牛津大学的研究人员奔向传染仍然在蔓延的地区,去南非小姆隆戈接种的医院,还有巴西和印度。对于疫情严重的美国,牛津大学也在考虑有争议的“挑战性试验”,即让年轻健康的志愿者接种疫苗,然后有目的地感染冠状病毒,看看疫苗是否有效。
如果两种疫苗最终都宣告失败,阿斯利康的情况可能不会更糟。但全世界迫切渴望的“结束封锁”只会更加遥遥无期。

疫苗研发领军者
目前至少有168种新冠疫苗正在研发,当中一种或多种疫苗有望成功。以下6种疫苗格外有前景,都处于二期或三期试验中,也就说明早期研究中表现良好,目前正进行更大范围的安全性和有效性测试。
牛津/阿斯利康
该疫苗使用修改后的黑猩猩病毒,产生引发感染的新冠病毒表面蛋白质。抗原让身体产生免疫反应。早期试验显示,疫苗可增加抗体和T细胞;II期和III期试验正在进行中。
科兴
中国生物制药公司科兴的疫苗采用灭活的新冠病毒(大多数疫苗都采用该方法)。经过两轮试验,该疫苗似乎安全并产生了抗体。最近科兴在疫情蔓延的巴西启动了第三阶段研究。
辉瑞/BioNTech
美国制药巨头辉瑞和德国的BioNTech正在合作研发疫苗,主要使用指导细胞制造蛋白质的基因编码片段信使核糖核酸。目标是修改信使核糖核酸,促使细胞产生新冠病毒蛋白,从而引发免疫反应。得知早期试验结果后,美国政府投资19.5亿美元确保成功后优先获得疫苗。
Moderna
该初创公司位于马萨诸塞州剑桥,也使用基于信使核糖核酸的疫苗。(阿斯利康在Moderna 持有9%股份)该项目得到美国政府9.5亿美元资助。第一阶段试验中,45名接种者全部出现免疫反应。研究于7月底启动,招募了30000名志愿者。
康希诺
康希诺是专门生产疫苗的中国公司,一定程度上得到了美国制药公司礼来的支持,该公司正在测试基于腺病毒(引起感冒的病毒)基因突变的疫苗。中国军方已经批准在军队测试疫苗,更广泛的安全性试验正在进行中。
默多克儿童研究所
澳大利亚的默多克儿童研究所正在试验卡介苗(BCG),上世纪20年代研发出的抗结核药物。研究表明,卡介苗可以预防其他类型呼吸道感染;正在进行Ⅲ期试验,以确定免疫反应能否用于预防新冠病毒。(财富中文网)
本文另一版本登载于《财富》杂志2020年8/9月刊,标题为《东山再起的制药巨头引领行业对抗新冠病毒》。
译者:Feb
6月底一个星期三的早上,南非约翰内斯堡郊外黑人聚居的索韦托,小姆隆戈走进克里斯·哈尼·巴拉瓜纳医院。他坐在诊室里解开紫色夹克衫拉链,因为此时南半球正是冬天。为了避免感染新冠病毒,他一直戴着红色布口罩。
姆隆戈从外套袖子里伸出左臂,拉起衬衫袖口。护士把针扎进胳膊注射透明液体时,他有点紧张。“我有点害怕,但我想了解这种疫苗,然后告诉亲朋好友,”他对身旁围观的记者说。
等待答案不仅仅是姆隆戈,全世界都在等。这位年轻的志愿者是南非首批约2000名患者之一,测试由英国牛津大学科学家研发的新冠疫苗,英国、美国和巴西都有数千人参与。
疫苗是终结新冠病毒全球大流行的关键。如果成功,经济就能重归增长,也可以拯救无数生命。没有疫苗,数以百万计的人可能会失去生命,企业和政府也会一直笼罩在病毒的阴影中。
根据世界卫生组织的数据,截至7月底,至少有168种候选疫苗处于研发阶段。但牛津疫苗在人体试验方面可以说进展最快。初步的人体试验显示颇有希望。为了推动进展,美国政府提前订购了3亿剂,耗资10亿美元。英国订购了1亿剂,欧盟订购了4亿剂,还达成协议向发展中国家提供超过13亿剂。
救命一针:南非索韦托一家医院里的志愿者正在接受注射,这是牛津大学詹纳研究所开发的新冠疫苗临床试验一部分。如果试验成功,阿斯利康将批量生产数十亿剂。
尽管如此,疫苗研发始终是一场赌博。麻省理工学院在2018年的研究发现,传染病疫苗研发失败的几率达66%。英国制药巨头阿斯利康的首席执行官苏博科(Pascal Soriot)大手笔下注牛津研发的疫苗。4月底,苏博科果断出手,阿斯利康击败疫苗研发方面成绩更好的竞争对手,携手牛津成为新冠疫苗商业合作伙伴,争取在全球研发竞赛中领先。
与牛津合作引起了外界对阿斯利康的极大关注。但这只是过去八年里阿斯利康一系列大胆合作、战略转型和谨慎冒险举动的最新一步,正是因为各种举措阿斯利康才能从大药企里最不成功的药物研发公司发展为最可靠的巨头。苏博科是推动转型的工程师。这位61岁的药剂师出生于法国,虽然大部分职业生涯都在国外,英语里还是总带着法语味。他头发乌黑,与喜欢的清爽白色衬衫形成鲜明对比,他说话非常直率,在高管圈中相当少见。苏博科告诉《财富》杂志,他信奉“轻松又紧张”的氛围。“我们必须认真对待工作,但不应该把自己看得过重。”他说。
当然,很少有制药公司的首席执行官面临着新冠病毒一样严重的生存挑战。与牛津大学的合作存在风险,因此阿斯利康抽调数百名员工在疫苗被证实有效之前就扩大临床试验和生产。至于疫苗是否真的有效,要到今年秋天才能知道。但如果临床试验得到积极的结果,美国和英国监管机构有望紧急批准,苏博科承诺尽快在9月下旬开始供应,从而开始大规模接种。
平均来说,研发一款新疫苗需要10年以上的时间。这次却只有6个月。即使成功,去年收入244亿美元的阿斯利康一开始也不会赚钱。因为阿斯利康已经同意以成本价提供最开始的20亿剂疫苗,还有可能提供更多。只有证明新冠是流感一样地区性季节性的威胁,人们需要定期接种疫苗,才能提高阿斯利康的利润。不过,股市上阿斯利康已经暴涨。宣布与牛津合作的当天,公司股价飙升至有史以来最高。之后股价最高达到97英镑(124美元),比疫情前上涨了38%,目前阿斯利康是伦敦富时100指数里市值最高的公司。
至于阿斯利康如何获得牛津疫苗的内幕消息,跟所有商业故事一样也是靠关系。但同样关系到新冠病毒令人担心的地缘政治。这是非凡的企业转型故事,为关注科学前沿希望获利的企业以及想推进激进文化变革的高管提供了经验教训。
去年12月,中国武汉出现神秘呼吸道疾病的消息刚浮出水面,立刻引起了阿斯利康注意。与其他西方制药公司相比,阿斯利康在中国的业务规模更大。公司6.15万名员工中,近四分之一常驻中国,去年公司在华收入约48亿美元。从去年12月底开始,苏博科几乎每天都与公司驻中国的高管视频通话,跟踪疫情发展。
“我们很快就开始考虑,怎样才能提供帮助?”苏博科回忆道。阿斯利康购买了900万台可以过滤病毒的FFP3呼吸机,捐赠给世界各地的医护工作者。公司开始调查现有药物,包括抗炎症的白血病治疗手段和保护心脏和肾脏的糖尿病药物能否用于抑制新冠病毒。阿斯利康也与范德比尔特大学合作研究可能用于治疗的合成抗体。当英国检测新冠病毒能力明显缺乏时,阿斯利康与英国竞争对手葛兰素史克和剑桥大学合作成立了新的检测实验室。
阿斯利康的应对手段中明显缺少一块:疫苗。不过疫苗领域并不是阿斯利康的强项。目前公司只销售一种疫苗,预防季节性流感的鼻喷雾。但在离剑桥大学总部不远的对手大学里,事件即将出现变化。
詹纳研究所位于牛津大学一组研究实验室,坐落在低矮的现代化办公楼里,装有烟灰色玻璃窗和绿色保护层。研究所以18世纪第一位为人们接种天花疫苗的爱德华·詹纳医生名字命名,过去20年里,此地已经成为全世界最重要的疫苗研发中心之一。
牛津大学之所以能在冠状病毒疫苗研发中领先,主要因为本次的冠状病毒与引起非典(SARS)和中东呼吸综合症(MERS)的冠状病毒有相似之处。詹纳研究所的研究员莎拉·吉尔伯特一直在研究中东呼吸综合症疫苗,她很快意识到有可能用同样的方法治疗新冠病毒。
大多数疫苗都是用相应病毒降低毒性后制成。吉尔伯特的疫苗则有所不同,利用无害的黑猩猩病毒并采取基因改造,产生新冠病毒表面的刺突蛋白,从而使身体产生潜在的有效免疫反应。如此生产的疫苗至少有一项主要优势,即不必低温保存,这在缺乏可靠冷链运输和储存手段的发展中国家尤为重要。
牛津大学的科学家们通过先前的研究了解到,改良的黑猩猩病毒是安全的,也知道可以大批量生产。但协助监督牛津医学部与外部合作伙伴合作的教授约翰·贝尔说,牛津大学更深知需要大型制药公司帮助。“说明一点,为全世界制造数十亿剂量的疫苗,不是大学能做到或应该做的。”贝尔表示。68岁的贝尔是加拿大人,也是运动健将,习惯把眼镜顶在银灰色头发上。
牛津大学开始寻找了解疫苗的合作伙伴,但政治因素导致谈判变得困难。美国一些公司坚持获得全球独家生产权,牛津对此犹豫不决。特朗普政府准备提供大笔资金,确保美国公众能首先买到疫苗。美国、法国和德国等国政府都发誓阻止本国生产的疫苗出口。贝尔说,每年向牛津大学提供数百万英镑资金的英国政府越来越担心,最终可能得不到足够的疫苗接种,于是4月通知牛津大学寻找英国合作伙伴。
英国只有两家公司生产能力达标,就是葛兰素史克和阿斯利康。选择葛兰素史克似乎无需多考虑。葛兰素史克已经是全世界最大的疫苗生产商,拥有超过30种疫苗,包括麻疹、脑膜炎和肺炎疫苗。不过,葛兰素史克已经在自行研发新冠疫苗,包括与法国制药巨头赛诺菲和中国生物技术公司三叶草生物制药的合作。(与三叶草合作项目正处于早期人体试验阶段。)
阿斯利康之前几乎没有生产过疫苗。但其生产工艺与吉尔伯特改良黑猩猩病毒的过程颇为相似。(阿斯利康用来生产合成抗体。)更重要的是,贝尔与苏博科有私交。贝尔曾经在瑞士制药商罗氏的董事会任职,去年才离开。而苏博科曾经在罗氏表现优异,他在去阿斯利康担任最高职位之前曾经于罗氏担任两年首席运营官。“我知道苏博科追求最尖端的科学,也有勇气冒险。”贝尔说。“而且老实说,这种疫苗风险很高。”
牛津还考虑到了另一项重要因素:阿斯利康在苏博科的领导下研发领域的进展令人瞩目。“这是过去20年里制药公司东山再起的故事之一。”贝尔说。瑞银的股票分析师迈克尔·莱克敦斯在谈到阿斯利康的复苏时说:“很了不起。我以前从未见过,以后应该也难得一见。”
2012年,苏博科执掌阿斯利康时,发展前景相当暗淡。公司最畅销的高胆固醇药瑞舒伐他汀、治疗胃酸倒流的耐信和抗精神病药物喹硫平都即将跌落可怕的“专利悬崖”。一旦失去知识产权保护,仿制药制造商就能销售廉价的版本。阿斯利康年销售额的一半可能在五年内消失,金额达170亿美元。
更糟糕的是,公司在研发方面投入不足,无法推出新药代替原有畅销药。“当时,公司就像电子表格一样运转。”苏博科说。“整天想着节省成本增加利润,把利润用于回购,机械地提高股价。但公司没有前途。”很多投资者都同意。“他必须重新考虑战略。”当苏博科上任时,资产管理公司AGF的基金经理斯蒂芬妮·马厄这样表示。
苏博科刚一接手,就宣布停止回购,将资金投入研发。2011年,苏博科获任命前一年,阿斯利康的研发支出仅占销售额16%;到2019年,研发支出已占销售额26%,是同行当中最高的。不过,要想恢复药品生产线,不能遇到问题就投入资金解决,关键在于重新调整全公司的重点。
十年前,阿斯利康研发运作效率极低,只有4%的研发药物最终能面世。“每年在研发上花费50亿美元,却没有做出新药。”阿斯利康的生物制药研发负责人梅内·潘加洛斯说。2012年,咨询公司InnoThink生物制药创新研究中心的一项发现证据确凿,研究发现阿斯利康旗下获得美国食品和药监局批准的新药平均研发支出高达118亿美元,在业内是最差记录。
公司战略转变之前,“我们每年在研发上花费50亿美元,却没有做出新药。”阿斯利康的生物制药研发负责人梅内·潘加洛斯说。
苏博科的前任大卫·布伦南聘请潘加洛斯重振研究部门。苏博科接手后,加快了新药研发节奏。先前一次领导层会议上,一位销售主管说有位研究员的介绍“有点偏技术,我都听不懂。”苏博科公开提出指责。“如果你想成为高层领导,就要对我们的研究还有治疗的病人真正感兴趣然后真心投入。”潘加洛斯回忆道。“一片寂静,有几个人咽了咽口水。但如果是在场的研发人员,感觉相当好。”
改革刚刚起步就差点胎死腹中。2014年春,美国规模更大的制药商辉瑞发起了恶意收购。首席执行官回忆说,刚开始投资银行家最初告诉苏博科,公司保持独立的可能性不到10%。苏博科全力抵抗,并向投资者承诺,到2023年将维持股息增长,收入提高到400亿美元,增幅达75%。苏博科说,回想起来当时的收购激发了他的战略,也促使全公司清楚地认识到,要生存就要靠更有效也更能赚钱的研发。
阿斯利康一直主要研发初级保健药物,治疗过敏和高血压等慢性疾病。苏博科将重点转移到专科护理药物上,此类药要求保险公司提供高额补偿,利润率较高,尽管市场规模较小。
肿瘤学方面的调整最引人注目。在苏博科前任的领导下,阿斯利康逐步放弃癌症治疗领域。但之前公司外部重要的肿瘤学家告诉苏博科,阿斯利康公司即将停产治疗晚期卵巢癌的药利普卓可能畅销,因为临床试验结果显示有希望。于是苏博科调整方向,恢复了该药生产。
这是一个重大决定。利普卓是名为PARP抑制剂的药物先驱。此类药针对细胞修复至关重要的酶,通过阻断酶可以阻止癌细胞恢复活力。研究很快发现,利普卓也能够治疗部分胰腺癌、乳腺癌和前列腺癌。现在利普卓是最畅销的PARP抑制剂,2019年创造了12亿美元收入,销售额同比增长85%。其功能多样性也吸引了企业合作伙伴。2017年,默沙东同意向阿斯利康支付高达85亿美元,以共同研发利普卓。
利普卓是阿斯利康肿瘤战略的代表案例,在该战略的指导下,公司研发出相当专业的药物,价格也相当高。泰瑞沙针对特殊且高度危险的肺癌突变,已经成为阿斯利康最耀眼的明星,仅去年就带来32亿美元收入。英飞凡是治疗肺癌、泌尿癌和膀胱癌的免疫疗法,2019年收入15亿美元。(阿斯利康还就肿瘤药物寻求合作,与日本第一三共株式会社签署了两项数十亿美元的协议。今年7月,阿斯利康同意支付高达60亿美元,与第一三共合作开发可限制化疗副作用的药物。)肿瘤药目前占阿斯利康年销售额37%,超过其他领域,远高于五年前的占比12%。
除了肿瘤,苏博科还围绕心血管、肾脏、代谢疾病、呼吸系统疾病和免疫学药物重组了阿斯利康。各种类别中,苏博科和潘加洛斯建立了严格程序评估候选药物。该程序又称为“五R框架”,要求开发药物之前必须确定五个方向“准确”:准确的目标、准确的患者群体、准确的身体组织、准确的安全制度,以及最关键的准确商业机会。清单听起来像制药行业基础知识,但之前阿斯利康很缺乏相关纪律。
严格的部署大大减少了阿斯利康研发药物的数量。但由于将有希望的分子从临床前研究转移到完成晚期临床试验,成功率显著提高。之前成功率仅为4%,现在是20%,是行业平均水平的三倍。
阿斯利康还利用新数字技术重新设计临床试验方法。研发部门的首席数字健康官克里斯蒂娜·杜兰表示,Merlin的软件能协助更快选择试验地点。而且几分钟内Merlin就可以估算出试验成本(过去需要几天)。有个研究生态系统里,受试者往往严重偏向男性和白人,Merlin则帮助选择更能反映真实世界人口的试验人群。还有个叫“控制塔”的软件系统,管理人员可在数据面板上一次性获得阿斯利康所有相关试验的快照,从而预测招募患者时可能出现的问题。“之前的系统我们已经用了20年,过去两年里全盘改变,很疯狂也很勇敢,但最后成功了。”杜兰说。
为了强调以科学为导向,2016年苏博科将位于伦敦的公司总部和位于麦克斯菲尔德的研究总部迁至剑桥。苏博科正在监督建设造价12亿美元的战略研发中心地标建筑,外形为圆形玻璃甜甜圈,可以展示阿斯利康科学家的工作。建筑距离被称为“诺贝尔工厂”(已经荣获12次)的政府分子生物学实验室,还有顶尖的癌症和干细胞研究实验室只有几步之遥。迁址以来,阿斯利康离开与剑桥大学的合作从五年前的不到10个增加到130多个。
如今,阿斯利康公司的规模比改造前要小,主要因为原先畅销药失去专利保护后的销售损失。去年,阿斯利康收入比2011年下降了27%。但投资者似乎认为,药品质量弥补了销售数量上的不足。自苏博科接手以来,以美元计算,阿斯利康的股价已经上涨137%,轻松跑赢同期标普医药精选行业指数61%的涨幅。
4月最后一周,阿斯利康生产牛津疫苗的交易在一连串电话会议中促成。牛津大学提出了两个条件:疫情结束之前阿斯利康放弃疫苗的利润,承诺尽可能广泛地提供疫苗。两项阿斯利康都同意。
对阿斯利康来说,疫苗是形象项目,可以证明公司有能力与科学前沿的学术团体和生物技术公司合作。这比吹嘘专利权更有价值,相关合作关系对阿斯利康的药品生产线至关重要。与此同时,疫苗项目速度也可以测试阿斯利康为推进更高效临床试验建立的系统。
南非索韦托临床试验中,一小瓶牛津大学/阿斯利康合作推出的试验性新冠疫苗。
为将通常需数年时间的测试浓缩在几个月内完成,牛津大学采取了前所未有的措施:以往第一阶段试验只招募少量病人,目的是通过小规模试验证明药物安全,本次牛津大学招募了1100人,并加快了接种速度。第二阶段的试验是在更多志愿者身上进行安全性和有效性试验,第三阶段试验证明药物效果,三个阶段通常按顺序进行。本次则是同时进行。牛津在英国招募了10000人,还有巴西的5000人和南非的2000人,阿斯利康在美国也招募了30000名志愿者。
苏博科说,预计结果将在9月至11月之间择时公布。但疫情紧迫性意味着试验结束之前就要开始大规模生产疫苗。在各国政府和国际机构资助下,阿斯利康不计利润授权全球企业生产,还与私营生物技术公司印度血清研究所达成里程碑式协议,为中低收入国家提供10亿剂。
6月初,阿斯利康飞涨的股价一度陷入恐慌。彭博新闻社援引匿名消息人士报道称,阿斯利康已经与美国吉利德制药公司商谈超大规模的合并,如果消息属实将成为制药行业历史上规模最大的并购。疫情中吉利德因旗下瑞德西韦家喻户晓,瑞德西韦是少数几款被证明可以减少新冠重症患者住院时间的药物之一。
吉利德拒绝就报道发表评论,苏博科也拒绝置评。不过,他在接受《财富》杂志采访时表示,当前环境下发起并购谈判意义不大。他说:“我只想请大家看清事实。”他还表示,疫情中推进临床试验让人费尽心思。疫苗研制也是如此。“这项任务让大家充满活力,但对很多人来说是艰巨的任务。”此外,考虑到旅行限制和隔离措施,谈判团队都无法面对面。“想象一下史上最大的制药行业并购案通过Zoom视频会议完成?开玩笑吧。”他说。
总体而言,业内人士和股市分析师都认为合并机会很小。不过有人指出,也有一种可能的情况下合并具有合情性,这种可能性会让投资者毛骨悚然,如果阿斯利康因为现金短缺而被迫交易,该怎么办?
吉利德坐拥240亿美元的现金储备。最近阿斯利康取得了成功,股票估值也很高,现金流却很差。去年,公司未能通过经营产生足够净现金支付35亿美元股息,而苏博科抗击辉瑞时承诺现金流可以继续保持增长。后来,公司不得不增发股票筹集35亿美元,这也是20年来阿斯利康为支付股息和履行其他义务首次增发。
阿斯利康现金短缺问题一定程度上源于推动创新。该公司重组费用终止遗留项目,向新药开发合作伙伴一次性预付款,举债为研发提供资金,种种策略都会影响现金流。伦敦Intron Health的分析师纳瑞许·秋汉表示,公司采用了“大量创新会计手段”,即合法但激进的策略,例如,合伙企业存续期间分期偿还一次性付款,防止相关交易降低阿斯利康让投资者注意的非公认会计准则下“每股核心收益”数字。
如此高空走钢丝,让一些投资者保持谨慎。瑞银的洛伊希腾对阿斯利康业绩扭转表示钦佩,但与其他两位分析师(在24位阿斯利康分析师中)对股票给予卖出评级。洛伊希腾称该公司为“概念股”。他说,投资者说服自己,阿斯利康搭建了“平台”生产一系列畅销药,但其表现并不能证明超常估值的合理性,目前公司股价高达每股收益的106倍。
苏博科指责像洛伊希腾之类批评者“眼光短浅到只能看见自己的鞋,看不到地平线。”他说:“人们必须决定的问题是,‘我相信这家公司的未来吗?’”他还为阿斯利康的股息辩护称,耐心的股东自会得到回报。他表示,现金流最终会有所改善。“很快,说‘你应该削减股息’的人转头就会改换批评论调,质问说,‘你为什么不增加股息?’ ”
目前,关于阿斯利康长期财务状况的争论都可以归于一个问题,牛津疫苗能否起效?
7月20日,医学专业杂志《柳叶刀》发表了牛津大学第一阶段试验的结果,答案出现一丝曙光。头条新闻的内容偏正面。(“牛津冠状病毒疫苗首次人体试验显示有望成功。”路透社宣称。),然而消息传出后,阿斯利康的股价下跌5.9%,创下自3月以来最大单日跌幅,随后继续走低。
第一阶段的试验是为了证明疫苗不会威胁使用者的健康,同时也可提供疫苗效果的线索。阿斯利康牛津疫苗似乎在两方面都取得了成功。该药没有严重的副作用。详细分析25名接受过单剂量疫苗接种的志愿者血液后发现,91%的志愿者产生了能中和病毒的抗体。第二次接受增强剂量的10名志愿者中,100%产生抗体,接种者体内抗体水平与感染过新冠病毒又恢复的患者体内抗体水平相当。
那么,市场为何感到不安?部分原因是,第二次使用了加强剂量。结果与第一组结果之间的差异可能说明牛津的疫苗需要两剂。正如潘加洛斯当时指出,大多数竞争中的疫苗研发也在考虑两剂方案。然而,如果需要多次注射,全球疫苗接种计划就会变复杂也更昂贵。如果有种疫苗只需注射一次就能产生免疫效果,可能就会成为首选,从而抵消阿斯利康的先发优势。
牛津大学的疫苗也可能输给两款竞争对手的疫苗。分析人士阅读《柳叶刀》报告后指出,一些竞争疫苗的初步试验显示抗体生成水平高于牛津大学的疫苗,尤其是辉瑞公司与生物技术公司BioNTech合作开发的疫苗。另一个悬而未决的问题是:疫苗是否实现“杀菌免疫”,可以防止接种者将病毒传播给未接种的人。
基于种种原因,牛津大学的贝尔把疫苗成功率定在不超过50/50。“根本不是板上钉钉。”他说。
尽管如此,第一阶段的研究提供信任阿斯利康和牛津团队能成功的理由。抗体数相当高,令人鼓舞。而且到最后新冠病毒抗体可能不是疫苗有效性的唯一关键(事实上越来越多研究表明,抗体可能迅速消退)。阿斯利康和牛津团队积极指出,其疫苗也引发T细胞的强烈反应,T细胞会寻找并消灭病原体。潘加洛斯告诉记者,由于细胞战士表现优异,他“越发相信”牛津疫苗能提供至少一年免疫力。
至于与其他疫苗的强度对比,牛津大学和阿斯利康的研究人员指出,到目前为止,试验采用的血样分析方法各不相同,简单比较不太可能。此外苏博科观察到,市场空间可以容纳不只一个赢家,毕竟要实现全世界安全接种肯定需要多款疫苗。
事实上,牛津大学并不是阿斯利康在新冠疫苗方面的唯一赌注。苏博科出任首席执行官后不久,阿斯利康就向马萨诸塞州剑桥一家生物技术初创公司Moderna投资了2.4亿美元。后来阿斯利康追加了1.4亿美元投资,持股比例增至9%。Moderna正在开发基于信使核糖核酸(mRNA)的疫苗,信使核糖核酸负责将指令从细胞核传达到制造蛋白质的部分。修改信使核糖核酸后,就可以让细胞产生想要的蛋白质,包括(有希望)引起免疫反应的蛋白质。Moderna的疫苗已经进入大规模人体试验阶段,阿斯利康的持股价值也飙升至30多亿美元。
如果运气好的话,两种疫苗都可能成功。现在所有目光都集中在后期试验上。测试需要在新冠病毒感染猖獗的地方进行,科学家就可以比较接种过疫苗的群体和对照组。这就是为何牛津大学的研究人员奔向传染仍然在蔓延的地区,去南非小姆隆戈接种的医院,还有巴西和印度。对于疫情严重的美国,牛津大学也在考虑有争议的“挑战性试验”,即让年轻健康的志愿者接种疫苗,然后有目的地感染冠状病毒,看看疫苗是否有效。
如果两种疫苗最终都宣告失败,阿斯利康的情况可能不会更糟。但全世界迫切渴望的“结束封锁”只会更加遥遥无期。
疫苗研发领军者
目前至少有168种新冠疫苗正在研发,当中一种或多种疫苗有望成功。以下6种疫苗格外有前景,都处于二期或三期试验中,也就说明早期研究中表现良好,目前正进行更大范围的安全性和有效性测试。
牛津/阿斯利康
该疫苗使用修改后的黑猩猩病毒,产生引发感染的新冠病毒表面蛋白质。抗原让身体产生免疫反应。早期试验显示,疫苗可增加抗体和T细胞;II期和III期试验正在进行中。
科兴
中国生物制药公司科兴的疫苗采用灭活的新冠病毒(大多数疫苗都采用该方法)。经过两轮试验,该疫苗似乎安全并产生了抗体。最近科兴在疫情蔓延的巴西启动了第三阶段研究。
辉瑞/BioNTech
美国制药巨头辉瑞和德国的BioNTech正在合作研发疫苗,主要使用指导细胞制造蛋白质的基因编码片段信使核糖核酸。目标是修改信使核糖核酸,促使细胞产生新冠病毒蛋白,从而引发免疫反应。得知早期试验结果后,美国政府投资19.5亿美元确保成功后优先获得疫苗。
Moderna
该初创公司位于马萨诸塞州剑桥,也使用基于信使核糖核酸的疫苗。(阿斯利康在Moderna 持有9%股份)该项目得到美国政府9.5亿美元资助。第一阶段试验中,45名接种者全部出现免疫反应。研究于7月底启动,招募了30000名志愿者。
康希诺
康希诺是专门生产疫苗的中国公司,一定程度上得到了美国制药公司礼来的支持,该公司正在测试基于腺病毒(引起感冒的病毒)基因突变的疫苗。中国军方已经批准在军队测试疫苗,更广泛的安全性试验正在进行中。
默多克儿童研究所
澳大利亚的默多克儿童研究所正在试验卡介苗(BCG),上世纪20年代研发出的抗结核药物。研究表明,卡介苗可以预防其他类型呼吸道感染;正在进行Ⅲ期试验,以确定免疫反应能否用于预防新冠病毒。
本文另一版本登载于《财富》杂志2020年8/9月刊,标题为《东山再起的制药巨头引领行业对抗新冠病毒》。
译者:Feb
On a Wednesday morning in late June, Junior Mhlongo arrived at the Chris Hani Baragwanath hospital in Soweto, the predominantly Black township outside Johannesburg, South Africa. Sitting inside an examination room, he unzipped the purple jacket he wore to guard against winter in the southern hemisphere. But he kept on the red cloth mask he wore to guard against the spread of COVID-19.
Mhlongo slipped his left arm from his coat sleeve and hiked up the cuff of his shirt. He winced as a nurse stuck a needle into his arm and injected him with a syringe of clear liquid. “I feel a little bit scared, but I want to know what is going on with this vaccine, so that I can tell my friends and others,” he told the reporters who had come to watch.
It’s not just Mhlongo: The whole world is awaiting an answer. The young volunteer is among the first of some 2,000 patients in South Africa—and thousands more in the U.K., the U.S., and Brazil—participating in clinical trials for a vaccine for COVID-19, pioneered by scientists at the University of Oxford in England.
A vaccine is the key to ending the long, global nightmare of the coronavirus pandemic. It can unlock economies and save countless lives. Without it, millions more could die, and business and government will remain hostage to the virus.
As of late July, there were at least 168 different vaccine candidates in some stage of development, according to the World Health Organization. But the Oxford vaccine is arguably the furthest along in human testing. Preliminary human trials have been promising. In anticipation of success, the U.S. government has preordered 300 million doses at a cost of $1 billion. The U.K. has ordered 100 million; the European Union, 400 million. Deals have been struck to provide the developing world with more than 1.3 billion doses.
Still, vaccine development is always a gamble—a 2018 MIT study found that 66% of vaccine candidates for infectious diseases fail. And no one has staked more on the Oxford vaccine than Pascal Soriot, chief executive officer of AstraZeneca, the British pharma giant. Soriot scored a coup in late April when AstraZeneca swept in to become Oxford’s commercial partner on its COVID-19 vaccine, displacing rivals with better track records in vaccine development to seize the lead in this global race.
The Oxford partnership has drawn unprecedented attention to AstraZeneca. But it’s only the latest in a series of bold collaborations, strategic shifts, and calculated risks at the company over the past eight years—moves that have elevated AstraZeneca from one of Big Pharma’s least successful drug developers to its most dependable. Soriot is the engineer of that metamorphosis. Born in France, the 61-year-old pharma lifer has spent most of his career abroad but still speaks in French-inflected English. He has jet-black hair that contrasts with the crisp white dress shirts he favors, and he speaks with a directness rare in executives of any nationality. Soriot tells Fortune he believes in “casual intensity.” “We have to take what we do seriously, but we should not take ourselves too seriously,” he says.
Of course, few pharma CEOs have faced a challenge as existentially serious as COVID-19. The Oxford partnership is risky, requiring AstraZeneca to commit hundreds of staffers to expanded clinical trials and manufacturing—long before the vaccine is even proven. Whether the vaccine is truly effective won’t be known until sometime this fall. But if clinical trials show a positive result, U.S. and U.K. regulators are expected to approve the vaccine on an emergency basis, and Soriot has promised to have doses ready as soon as late September so mass vaccination programs can begin.
On average, developing a new vaccine takes more than a decade. In this case, it is being done in about six months. Even if it works, AstraZeneca, which brought in $24.4 billion in revenue last year, will make no money at first. It has agreed to provide most of those initial 2 billion doses, and perhaps billions more, at cost. The vaccine will boost AstraZeneca’s bottom line only if COVID-19 proves to be an endemic, seasonal menace, like the flu, for which people require regular vaccinations. Still, AstraZeneca (or AZ, as it is informally known) has seen a stock market bump. On the day the Oxford deal was announced, its shares soared to an all-time peak. They have since traded as high as 97 pounds ($124), up 38% from their pre-pandemic price, and AZ now commands the highest market capitalization on London’s FTSE 100 index.
How AstraZeneca got the inside track on the Oxford vaccine is a story, like all business stories, about relationships. But it’s also about COVID-19’s fraught geopolitics. And it’s a tale of a remarkable corporate turnaround—one that holds lessons for any business trying to profit at science’s cutting edge, and for any executive trying to lead through a radical cultural transformation.
****
When news of a mysterious respiratory illness in Wuhan, China, surfaced in December, AstraZeneca was quick to take notice. More than any other Western pharmaceutical firm, AZ has bet big on China. Almost a quarter of its 61,500 people are based there, and last year its Chinese revenues were some $4.8 billion. Beginning in late December, Soriot held almost daily video calls with AstraZeneca’s Chinese executives, tracking the outbreak as it inched toward becoming a pandemic.
“Then we quickly thought, ‘What can we do to help?’ ” Soriot recalls. AstraZeneca bought 9 million FFP3 respirators, which can filter out the virus, and donated them to health care workers worldwide. It began investigating whether any of its existing drugs—including a leukemia treatment that fights inflammation and a diabetes drug that can protect the heart and kidneys—could be repurposed to battle COVID-19. It is collaborating with Vanderbilt University on synthetic antibodies that might treat the disease. And after it became apparent that the U.K. lacked adequate COVID-19 testing capacity, AstraZeneca teamed up with its British rival, GlaxoSmithKline (GSK), and the University of Cambridge to set up a new test-processing lab.
One thing was conspicuously absent from AstraZeneca’s response: a vaccine. AZ is not a significant player in that field. It currently sells just one vaccine, a nasal spray that protects against seasonal influenza. But events taking place not far from its Cambridge, England, headquarters, in a rival college town, were about to change that.
Amid a cluster of research labs in Oxford, the Jenner Institute is housed in a low-slung modern office building with smoky glass windows and green cladding. Named for Edward Jenner, the 18th-century physician who first inoculated people against smallpox, it has emerged in the past two decades as one of the world’s foremost centers for vaccine development.
Oxford was able to leap out in front in the sprint for a coronavirus vaccine because of similarities between SARS-CoV-2, the coronavirus that causes COVID-19, and those that cause severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS). Sarah Gilbert, a Jenner researcher, had been working on a MERS vaccine, and she realized almost immediately it might be possible to use the same method for SARS-CoV-2.
The majority of vaccines are made using a weakened version of the virus they target. Gilbert’s vaccine is different: It takes a harmless chimpanzee virus and genetically modifies it to produce the surface spike protein found on SARS-CoV-2, allowing the body to develop a potentially effective immune response. Vaccines produced this way have at least one major advantage: They don’t have to be kept at subfreezing temperatures, which is especially important in developing countries that lack reliable cold transport and storage.
The Oxford scientists knew from previous work that the modified chimpanzee virus was safe. And they knew it could be manufactured in large quantities. But John Bell, the professor who helps oversee the Oxford medical-science division’s work with external partners, says the university also knew it would need help from Big Pharma. “Making billions of doses of vaccine for the whole world, let’s be clear, that’s not what universities could or should be doing,” says Bell, an athletic 68-year-old Canadian who habitually keeps his eyeglasses propped atop his silver-gray hair.
Oxford set out to find a partner with proven vaccine expertise—but politics made negotiations difficult. Oxford balked at some U.S. companies’ insistence on exclusive worldwide manufacturing rights. The Trump administration was prepared to offer large sums to guarantee the American public was first in line for a vaccine. Various governments, including those of the U.S., France, and Germany, vowed to block exports of vaccines made in their countries. Ultimately, Bell says, the British government, which steers millions of pounds in funding to Oxford each year, grew anxious that it could wind up without adequate vaccine access, and in April it told Oxford to find a British partner.
There were only two U.K. companies with sufficient manufacturing capability: GSK and AstraZeneca. GSK might seem like the obvious choice. It is the world’s top vaccine producer, with a portfolio of more than 30, including inoculations for measles, meningitis, and pneumonia. But GSK was already working on its own COVID-19 vaccine efforts, including one with French drug giant Sanofi and another with Chinese biotech firm Clover Biopharmaceuticals. (The project with Clover is now in early-stage human testing.)
AstraZeneca had virtually no vaccine track record. But it had experience with a manufacturing process similar to the one Gilbert uses for her modified chimp virus. (AZ uses it to make synthetic antibodies.) What’s more, Bell had a personal connection to Soriot: Until last year, Bell served on the board of Swiss drugmaker Roche, where Soriot had been a fast-rising star, serving as chief operating officer for two years before taking the top job at AZ. “I knew Pascal was somebody who was absolutely committed to the highest-quality science, but also prepared to take risks,” Bell says. “And, to be crystal clear, this vaccine is a big risk.”
Oxford also had another important factor in mind: AstraZeneca’s remarkable R&D rebound under Soriot’s leadership. “It’s one of the great turnaround stories in pharmaceutical companies over the past 20 years,” Bell says. Michael Leuchten, an equity analyst for Swiss bank UBS, says of AstraZeneca’s recovery: “It’s remarkable. I’ve never seen anything like that before, and I don’t think we will again anytime soon.”
****
When Soriot took the helm of AstraZeneca in 2012, the company faced a bleak future. Its bestselling medicines—Crestor for high cholesterol, Nexium for acid reflux, and antipsychotic drug Seroquel IR—were about to plunge off the industry’s dreaded “patent cliff.” As soon as they lost intellectual-property protection, generic drug manufacturers would be able to sell cheap copies. Half of AZ’s annual sales—$17 billion in total—were likely to disappear in the next five years.
Worse, the company, having underinvested in R&D, had no pipeline to replace its blockbusters. “At the time, the company was run like a spreadsheet,” Soriot says. “It was all about cost savings, increase the profit and use that for a buyback, and mechanically increase the share price. But the company was on the road to nowhere.” Many investors agreed. “He has to rethink the strategy,” Stephanie Maher, a fund manager at asset management firm AGF, said when Soriot was appointed.
The minute Soriot took over, he stopped the buybacks and plowed money back into science. AZ’s R&D spending as a percentage of sales was 16% in 2011, the year before Soriot was appointed; in 2019, it was 26%, the highest percentage of any of its peers. And reviving the drug pipeline wasn’t just a case of throwing money at a problem: It involved refocusing the entire company.
A decade ago, AZ’s R&D operation was extremely inefficient: Just 4% of its drug candidates made it to market. “We were spending $5 billion a year on R&D and not delivering any medicine,” says Mene Pangalos, who heads AZ’s biopharmaceuticals R&D. A damning 2012 analysis from the InnoThink Center for Research in Biomedical Innovation, a consultancy, found that AZ was spending, on average, $11.8 billion in R&D for every drug that received U.S. Food and Drug Administration approval, the worst record in the industry.
Soriot’s predecessor, David Brennan, hired Pangalos to revive the research division. After Soriot took over, he accelerated the effort to restock the medicine cabinet. In one early leadership meeting, a sales executive said a researcher’s presentation “had been a bit technical and over my head.” Soriot publicly upbraided him. “If you want to be a senior leader in our organization, you need to be interested and engaged in the science that we do and the patients that we are treating,” Pangalos recalls Soriot saying. “There was stunned silence and quite a few gulps. But if you were an R&D person in the room, it was the best we’d ever felt.”
The fledgling overhaul was almost smothered in its nest. In the spring of 2014, the larger U.S. drugmaker Pfizer launched a hostile takeover attempt. Investment bankers initially told Soriot the company had a less than 10% chance of remaining independent, the CEO recalls. Soriot repelled the effort with hefty promises to investors—pledging to sustain dividend growth and boost revenue to a lofty $40 billion, a 75% increase, by 2023. In retrospect, Soriot says, the takeover attempt galvanized his strategy, making it crystal clear to the entire company that its survival depended on more effective, and thus more profitable, R&D.
AstraZeneca had been focused on primary-care drugs, medications for chronic conditions such as allergies and high blood pressure. Soriot shifted its emphasis to specialty care—drugs that command high reimbursements from insurers and better profit margins, even though their market size is smaller.
Nowhere has that change in priorities been more dramatic than in oncology. Under Soriot’s predecessor, AstraZeneca had been divesting from cancer treatments. But early on, leading oncologists outside the company told the CEO that AZ was about to write off a potential blockbuster in Lynparza, a drug for late-stage ovarian cancer that had shown promise in clinical trials. Soriot reversed course and revived the drug.
It was a momentous decision. Lynparza emerged as a pioneer in a class of drugs called PARP inhibitors. These drugs target enzymes critical to cell repair; by blocking them, they keep cancer cells from rejuvenating. It soon emerged that Lynparza could treat some pancreatic, breast, and prostate cancers, too. It’s now the bestselling PARP inhibitor: In 2019, it generated $1.2 billion in revenue, with sales growing 85% year over year. Its versatility also makes it attractive to corporate partners. In 2017, Merck agreed to pay AZ up to $8.5 billion to share in Lynparza’s development.
Lynparza is the flagship example of AstraZeneca’s oncology strategy, which has yielded highly specialized medications that merit premium prices. Tagrisso, which targets a specific, highly dangerous mutation of lung cancers, has become AZ’s brightest star: It brought in $3.2 billion last year. Imfinzi, an immunotherapy treatment for lung, urinary, and bladder cancers, made $1.5 billion in 2019. (AZ has also struck partnerships for oncology drugs, including two multibillion-dollar deals with Japan’s Daiichi Sankyo in as many years. In July, it agreed to pay as much as $6 billion for the right to codevelop a Daiichi drug that could limit chemotherapy’s side effects.) Oncology now accounts for 37% of AZ’s annual product sales, more than any other area, and up from just 12% five years ago.
In addition to oncology, Soriot reorganized AstraZeneca around drugs for cardiovascular, renal, and metabolic disease, respiratory illness, and immunology. Across all those categories, Soriot and Pangalos instituted a rigorous process for assessing drug candidates. Known as “the five R framework,” it mandates that before the company commits to developing a drug it must be satisfied it has identified five “rights”: the right target, the right patient group, the right body tissue, the right safety regime, and, crucially, the right commercial opportunity. The checklist sounds like Pharma 101, but it’s the kind of discipline AstraZeneca had once lacked.
Disciplined deployment of the framework has drastically reduced the number of compounds AstraZeneca had under development. But it has lifted its success rate in moving promising molecules from preclinical investigation through to completion of late-stage clinical trials. Once a paltry 4%, that rate is now 20%, three times the industry average.
AZ has also used new digital technologies to reinvent its approach to clinical trials. Software called Merlin enables AZ to select trial sites 70% faster than before, according to Cristina Duran, chief digital health officer for the R&D division. Merlin produces trial cost estimates in minutes (it used to take days). In a research ecosystem where trial volunteers often skew disproportionately male and white, Merlin helps select trial populations that better reflect real-world demographics. Another software system, called Control Tower, allows managers to get a visual snapshot of all AZ’s trials on a single dashboard, helping them predict problems in patient recruitment. “We’ve taken systems that the company was using for 20 years, and in the past two years we changed them—which is either insane or brave, but it has been successful,” Duran says.
To cement its science-led ethos, in 2016 Soriot moved both AZ’s corporate headquarters, which had been in London, and its research headquarters, which had been in Macclesfield, to Cambridge. Here Soriot is overseeing construction of a signature, $1.2 billion strategic R&D center, a round glass doughnut to showcase the work of AZ’s scientists. The building is steps from the government molecular biology lab known as “the Nobel factory”—its scientists have won 12 of them—as well as top-flight cancer and stem cell research labs. Since moving, AZ has increased its collaborations with Cambridge University to more than 130, up from fewer than 10 five years ago.
AstraZeneca is a smaller company today than it was before its reinvention, thanks largely to the loss of sales from those older blockbusters that fell off the patent cliff. AZ’s revenues last year were 27% lower than in 2011. But investors seem to think the company has made up in drug quality for what it lacks in sales quantity. AZ’s stock price has risen 137% in dollar terms since Soriot took over, trouncing the 61% increase in the S&P Pharmaceuticals Select Industry Index over the same period.
****
AZ’s deal to produce Oxford’s vaccine was brokered in a flurry of Zoom calls during the last week of April. Oxford had two main conditions: that AstraZeneca forgo any profit on the vaccine until the pandemic is over, and that it commit to making the vaccine as widely available as possible. AZ agreed to both.
For AstraZeneca, the vaccine is a showcase project where it can demonstrate its ability to be a partner for academic groups and biotech companies at the forefront of science. That’s worth more than bragging rights: Those relationships are critical to feeding AZ’s pipeline of winning medicines. The speed of the vaccine project, meanwhile, is a key test of the systems AstraZeneca has built to run clinical trials more efficiently.
To condense testing that would normally take years into months, Oxford took unprecedented steps: Rather than recruiting just a few dozen patients for Phase I trials, which are designed to show a drug is safe by testing it on a small cohort, Oxford recruited 1,100 and accelerated the pace of inoculations. Phase II trials, which test for safety and efficacy on a much larger number of volunteers, and Phase III trials, which aim to prove that a drug is effective, would normally take place sequentially. Instead, they are happening simultaneously. Oxford is recruiting 10,000 people in the U.K. for this testing, plus 5,000 in Brazil and 2,000 in South Africa, while AZ is enrolling 30,000 volunteers in the U.S.
Results are expected anytime between September and November, Soriot says. But the pandemic’s urgency means large-scale vaccine production must start even before testing concludes. With funding from governments and international agencies, AZ has licensed manufacturing—again, at no profit—to firms across the globe, including a landmark deal with the Serum Institute of India, a private biotechnology company, to provide 1 billion doses for low- and middle-income countries.
****
In early June, AstraZeneca’s soaring stock was momentarily rattled. Bloomberg News, citing anonymous sources, reported that AZ had approached the U.S. pharmaceutical company Gilead about a supernova-scale merger—one that would be the largest in the sector’s history. The pandemic has made Gilead a household name, thanks to its production of remdesivir, one of the few drugs that has been shown to reduce hospitalization time for severely ill COVID-19 patients.
Gilead declined to comment on the report, as did Soriot. But, speaking in general terms to Fortune, he implied that initiating merger talks would make little sense in the current climate. “I would just invite you and everybody else to look at the facts,” he says. Figuring out how to execute clinical trials under pandemic conditions is an all-consuming undertaking, he says. So too is working on the vaccine. “It is energizing everybody, but it is a big job for a number of us.” Plus, given travel restrictions and quarantine measures, negotiating teams would be unable to meet face-to-face. “Imagine the biggest merger in the history of the industry, done by Zoom? I mean, come on,” he says.
Industry and stock market analysts, on the whole, discounted the possibility of a tie-up. Except, some noted, there was a possible scenario where the merger made sense—and it was a scenario that turned investors’ blood cold: What if AstraZeneca had to do a deal because it was running out of cash?
Gilead is sitting on a cash hoard of $24 billion. AZ, for all its recent success and highly valued stock, is cash poor. Last year the company didn’t generate enough net cash from operations to cover its $3.5 billion in dividend payouts—the ones Soriot promised to keep growing during his fight with Pfizer. In fact, the company had to raise $3.5 billion through an additional share offering, its first in 20 years, in order to meet that and other obligations.
AZ’s cash issues stem in part from its reinvention push. The company has taken restructuring charges to discontinue legacy projects, made lump-sum upfront payments to new drug-development partners, and taken on debt to fund R&D—all strategies that hurt cash flow today. Naresh Chouhan, an analyst at London’s Intron Health, an equity research boutique focused on health care, says the company has used “a lot of creative accounting”—legal but aggressive tactics, such as amortizing those lump-sum payments over the life of the partnership—to keep these transactions from depressing the non-GAAP “core earnings per share” figure AstraZeneca tells investors to pay attention to.
That high-wire act has left some investors wary. UBS’s Leuchten admires AZ’s turnaround, but he is also one of three analysts (out of 24 that cover AZ) to have a sell rating on the shares. Leuchten calls the company “a concept stock.” He says that investors have convinced themselves that AZ has a “platform” for churning out a succession of blockbusters, but that so far, its performance doesn’t justify its outsize valuation, currently 106 times its trailing earnings per share.
Soriot accuses critics like Leuchten of “looking at their shoes instead of looking at the horizon.” He says, “The question people have to decide about is, ‘Do I believe in the future of this company?’ ” He defends AZ’s dividends, saying they reward shareholders for their patience. Cash flow will eventually improve, he says. “And soon enough the people who say, ‘You should cut the dividend’ will move to another criticism: They will say, ‘Why won’t you increase the dividend?’ ”
****
For now, any debate about AstraZeneca’s long-term financial health is superseded by a single question: Will the Oxford vaccine work?
On July 20, the first glimmer of an answer emerged when medical journal The Lancet published the results of Oxford’s Phase I trials. The headlines were positive. (“First human trials of Oxford coronavirus vaccine show promise,” Reuters declared.) Yet AstraZeneca’s stock price fell 5.9% on the news, its largest one-day drop since March, and subsequently drifted lower still.
Phase I trials are designed to prove a vaccine doesn’t threaten users’ health, but they also offer clues to its effectiveness. The AZ-Oxford vaccine appeared to succeed on both counts. The drug showed no serious side effects. Of the 25 volunteers vaccinated with a single dose whose blood was analyzed in detail, 91% produced antibodies capable of neutralizing the virus. In 10 volunteers who also received a second booster dose, 100% produced antibodies—and in those subjects, antibody levels equaled those found in patients who recover from COVID-19.
So what unnerved the markets? In part, it was that booster-dose group. The disparity between their results and the first group’s may indicate that Oxford’s vaccine will require two doses. As Pangalos pointed out at the time, most competing vaccine efforts are also looking at two-dose protocols. Still, the need for multiple shots would make any global vaccination program more fiendishly complicated and expensive. If another vaccine could confer immunity with a single dose, it could become the preferred option, negating AZ’s first-mover advantage.
The Oxford candidate could also lose out to a rival two-dose vaccine. Analysts noted after the Lancet report that initial trials of some competing vaccines—notably, one that Pfizer is developing with biotechnology firm BioNTech (see box)—indicated higher levels of antibody production than Oxford’s. Another question hovering over the effort: Whether it will confer “sterilizing immunity,” which would keep inoculated people from spreading the virus to the unvaccinated.
For those reasons and more, Oxford’s Bell has put his vaccine’s odds of success at no better than 50/50. “This is not a slam dunk, at all,” he says.
Still, the Phase I study provided reason to believe AZ and Oxford could score. The antibody counts were encouragingly high. And COVID-19 antibodies may not be the only key to any eventual vaccine’s effectiveness (indeed, an increasing number of studies indicate that those antibodies may fade rapidly). AstraZeneca and Oxford were keen to note that their vaccine also prompted a strong response from T cells, which seek out and destroy pathogens. Thanks in part to these cellular warriors, Pangalos told reporters, he is “increasingly confident” Oxford’s vaccine will provide immunity for at least a year.
As for its strength relative to other vaccines, Oxford and AstraZeneca researchers note that, so far, no two of the trials have used an identical blood sample assay, making apples-to-apples comparisons impossible. What’s more, Soriot observes, there’s room for more than one winner: Safely inoculating the entire world will certainly require multiple vaccines.
And as it turns out, Oxford is not AZ’s only ticket in the COVID-19 vaccine sweepstakes. Shortly after Soriot became CEO, AstraZeneca invested $240 million in a biotechnology startup in Cambridge, Mass., called Moderna; AZ later invested a further $140 million, upping its stake to 9%. Moderna is developing a vaccine based on messenger RNA (mRNA), which carry instructions from a cell’s nucleus to parts of the cell that manufacture proteins. Modify mRNA and you can get the cell to produce whatever proteins you want—including ones that (hopefully) elicit an immune response. Moderna’s vaccine is now entering large-scale human testing, and its stock has soared on the news—inflating the value of AstraZeneca’s stake to more than $3 billion.
With a bit of luck, both vaccines might succeed. Now all eyes are on those later-stage trials. The testing needs to happen in places where coronavirus infections are rampant, so that scientists can make a comparison between inoculated individuals and a control group. That’s why Oxford researchers are racing to hotspots where cases are still surging—to Junior Mhlongo’s hospital in South Africa, as well as to Brazil and India and, yes, to the still-stricken U.S. Oxford is also contemplating a controversial “challenge trial”—where healthy young volunteers will be inoculated and then purposefully infected with the coronavirus to see if the vaccine works.
If both vaccines fail, AstraZeneca may not be materially worse off. But the world will be that much further away from obtaining the get-out-of-lockdown card it so desperately needs.
****
Leaders of the vaccine pack
At least 168 COVID-19 vaccine candidates are now in development—a fact that hopefully increases the likelihood that one or more will succeed. Here are six that look particularly promising. All are in Phase II or Phase III trials, which means they did well in early studies and are now being tested in larger groups for safety and efficacy.
Oxford/AstraZeneca
This vaccine uses a modified chimpanzee virus to produce a protein found on the surface of SARS-CoV-2, the virus that causes COVID-19. That antigen prompts the body to develop an immune response. In early trials, it increased production of antibodies and T cells; Phase II and III trials are underway.
Sinovac
This Chinese biopharma company bases its vaccine on an inactivated version of SARS-CoV-2 (the approach used in the majority of vaccines). The drug has appeared safe and produced antibodies in two rounds of trials. Sinovac recently launched Phase III studies in Brazil, where the virus has spread rapidly.
Pfizer/BioNTech
U. S. pharma giant Pfizer and Germany’s BioNTech are developing a vaccine that relies on messenger RNA (mRNA), bits of genetic code that instruct cells to make proteins. The goal: Modify mRNA so it prompts cells to make proteins that look like COVID-19, eliciting an immune response. Early trial results led the U.S. government to put up $1.95 billion to secure doses if the vaccine works.
Moderna
The Cambridge, Mass., startup also uses a vaccine based on mRNA. (AstraZeneca owns a 9% stake in Moderna.) The project has been backed by $950 million in U.S. government funding. The vaccine provoked an immune response in all 45 patients tested in a Phase I trial; a study involving 30,000 volunteers launched in late July.
Cansino
A Chinese company that specializes in vaccines and is partly backed by U.S. drugmaker Eli Lilly, Cansino is testing a vaccine based on a genetic mutation of an adenovirus (the kind that causes colds). The Chinese military has approved the vaccine for use in its ranks; wider safety trials are in progress.
Murdoch Children's Research Institute
This Australian institution is experimenting with the bacillus Calmette-Guérin (BCG) vaccine—a drug introduced in the 1920s to fight tuberculosis. Studies have suggested that BCG protects against other respiratory infections; Phase III trials are underway to see whether that immune response extends to COVID-19.
A version of this article appears in the August/September 2020 issue of Fortune with the headline “A reborn pharma giant takes the lead against COVID-19.”






